Who's Owning What? The Real Difference Between Product, Clinical Product and Clinical Directors
Your weekly newsletter on all things clinical product and building better healthcare 🏥
This is Clinical Product Thinking 🧠, a weekly newsletter featuring practical tips, frameworks and strategies from the frontlines of clinical product. I try to make this your best Sunday afternoon email. If you enjoy it, please hit the 💜 button.
Good afternoon friends, this is issue No. 008. Today we’re diving into a topic every scaling health-tech team wrestles with: who’s doing what and how to make it work.
Most health-tech teams can tell you what a Product Manager does.
Fewer can explain what a Clinical Director actually owns.
And almost no one knows where the Clinical Product Manager fits in that mix.
That gap matters because every time a founder hires “a clinical person” without clarity, they end up with blurred ownership, slow decisions and one very tired clinician wearing too many hats.
So let’s fix that.
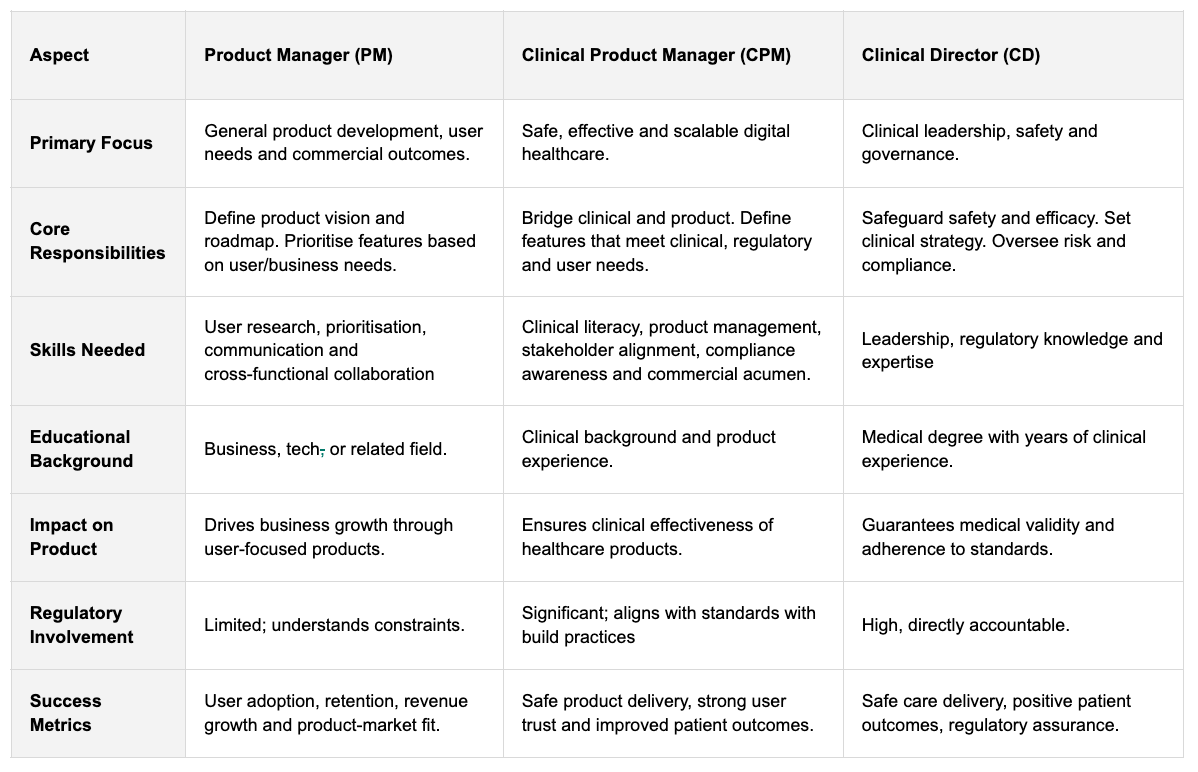
The Three Roles, at a Glance
Product Manager (PM) → Owns the what. Defines priorities and aligns product direction with user needs and business goals.
Clinical Product Manager (CPM) → Owns the how (safely). Translates clinical realities into product roadmaps, ensures decisions hold up to regulation, risk and patient impact.
Clinical Director (CD) → Owns the clinical integrity. Leads governance, protocols and safety oversight. They have the final sign-off when stakes are high.
And because I love a good comparative overview, here’s how that might break down across the key aspects of each role. (Reverse pinch to zoom on mobile!)
In theory, but not in practice
Even though I love to put things in neat boxes, in practice, it’s often a continuum.👇
Early-stage health-tech is messy. Everyone’s wearing six badges and half the systems are duct-taped together. But over time, those overlaps turn from helpful to hazardous.
When Blurry Becomes Risky
At Series A, “everyone does everything” might feel scrappy and efficient.
By Series B, it’s chaos disguised as collaboration.
If no one knows who’s accountable for clinical governance, guess who the regulator will decide it was? Likely the person with “clinical” in their title.
That’s why CPMs need guardrails:
Stay within scope. Influence protocols, don’t author them solo unless you’re qualified and approved.
Refuse phantom accountability. If you’re carrying out daily risk decisions without documented oversight, you’re likely de facto accountable, and that’s a governance hole waiting to surface.
What the Clinical Director Owns (and You Probably Shouldn’t)
Usually, the Clinical Director is responsible for the following. If you’re a CPM and you find yourself owning these, pause and think.
Clinical Oversight & Decision-Making — Holds final authority on clinical protocols, especially where evidence or precedent is limited. Reviews and approves high-risk or exceptional cases to safeguard patient outcomes
Governance & Risk — Owns the organisation’s clinical risk and governance frameworks. Chairs or advises governance committees to ensure safe, consistent and evidence-based care.
Regulatory & Legal Compliance — Ensures alignment with statutory medical standards (GMC, NICE, or international equivalents). Serves as the primary clinical contact during audits, inspections or investigations.
Education & Leadership — Sets clinical standards, mentors senior clinicians and drives ongoing professional development across teams.
Ethical Oversight — Acts as final arbiter for complex or grey-area care decisions, maintaining integrity and patient trust.
Research & Innovation — Approves or sponsors clinical research, ensuring ethical design and alignment with strategic objectives.
It’s worth noting some Clinical Product Managers bring such deep clinical experience that, depending on the product and business model, they’re qualified to assume elements of the Clinical Director remit.
Why Role Clarity Isn’t Bureaucracy
All this talk of lanes and titles might sound like people refusing to share their Legos.
But role clarity doesn’t slow innovation; it enables it.
When PMs, CPMs, and CDs each know their swim lane:
Decisions happen faster.
Feedback loops tighten.
Teams trust the process.
Patients stay safer.
It’s not hierarchy. It’s effective risk management.
The Common Goal
Different lenses, same mission: build products that improve care without breaking trust.
PMs champion user and business value.
CPMs bridge clinical reality and product delivery.
CDs protect clinical integrity.
Together, they create the balance that makes health-tech actually work: safe, scalable and human.
Join the next event 🎇
📆 12th November - Clinical Product Dinner: Regulation Without Killing Innovation
With Dr Dom Pimenta, CEO & Founder of Tortus AI, empowering clinicians with advanced AI through their Class I medical device. Sign up here. (Will sell out!)
Hiring Spotlight 🚀
Hertility Health is hiring their first Clinical Product Manager.
I caught up with Tulsi Patel, Director of Product, on this role and her approach to building the clinical product function is refreshingly forward-thinking. 👉 Apply here
That’s all for this week. See you next time! 👋
🤝 Work with me | 📅 Attend an event | | ✍️ Send a message
Written by Dr.Louise Rix, Head of Clinical Product, doctor and ex-VC. Passionate about all things healthcare, healthtech and clinical product (…obviously). Based in London. You can find me on Linkedin.
Made with 💜 for better, safer HealthTech.